If the amounts of two or more reactants are given it is a limiting reactant problem Step 2. The easiest way is to convert both reactant quantities into moles of the particular product that we are solving for.
 Limiting Reactant Mol Mol Method A Youtube
Limiting Reactant Mol Mol Method A Youtube
Calculate the moles of a product formed from each mole of reactant.
Quick way to determine limiting reactant. The answer is less than 111 the number of moles of magnesium hydroxide so the magnesium hydroxide is in excess and the hydrochloric acid is the limiting reactant. Using the mole ration. Once you find the moles only convert one of them to the moles of the other reactant.
When youre dealing with equal masses of two reactants the reactant that has the greater molar mass will have the smallest number of moles present in the given mass. Using the product approach. There are two ways to determine the limiting reagent.
Identify the reactant giving the smaller number of moles of product. Students use a Pressure Sensor to experimentally determine the limiting and excess reactant when the amount of one reactant is varied and analyze data to reveal the coefficients in the balanced reaction. In most limiting reactant stoichiometry problems the real goal is to determine how much product could be formed from a particular reactant mixture.
CO 2H2 CH3OH Step 1. For the first method well determine the limiting reactant by comparing the mole ratio between and in the balanced equation to the mole ratio actually present. The limiting reactant or reagent can be determined by two methods.
One method is to find and compare the mole ratio of the reactants used in the reaction approach 1. Find the moles of each reactant present. 02072019 Determine which reactant is limiting by dividing the number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation.
In order to calculate the mass of the product first write the balanced equation and find out which reagent is. 15082020 The limiting reagent is the one that is totally consumed. Determine if it is a limiting reactant problem.
To determine the moles of ammonium chloride produced we need to find the limiting reactant. 10102018 Think about simple ratios when dealing with limiting reactant questions. To find the limiting reagent and theoretical yield carry out the following procedure.
Whichever substance turns up as the smallest number will be the limiting reactant. 10062020 If you define limiting reagent it is a reactant in a chemical reaction which determines the amount of product which is produced. The limiting reactant or limiting substrate is the reactant present in the smallest stoichiometric amount.
12102018 The way I calculate the limiting reactant is by first finding the amount of moles are in the reactants given. This will allow you to easily observe which one of the reactants produces the least amount of product and is therefore the limiting reactant. Supports NGSS Performance Expectation HS-PS1-7.
The smaller number will be the limiting reactant. 10092016 For starters a very quick way of figuring out which reactant will act as the limiting reagent when dealing with equal masses of the two reactants is to look at their molar masses. All of the methods give the same answer though so you can choose whichever approach you prefer.
While other reactants may be present in smaller absolute quantities at the time when the last molecule of the limiting reactant is consumed residual amounts of all reactants except the limiting reactant will be present in the reaction mixture. H2o is present in an unlimited amount so it cannot be the limiting reactant. Use mole ratios to calculate the number of moles of product that can be formed from the limiting reactant.
It limits the reaction from continuing because there is none left to react with the in-excess reactant. Use mathematical representations to support the claim that. 22052017 A quick way to determine limiting reagent or reactant is to convert all data dimensions to moles and divide each by the respective coefficient in the balanced Equation.
The quickest way to determine limiting reactant is to convert to moles and divide each mole value by the coefficient in thebalanced equation. What is a Limiting Reactant. In this case the mole ratio of and required by balanced equation is.
For example if you had a equation of 2h22o2----2h202 find the moles of h2 through o2 by multiplying the moles of o2 found to the moles of h2 over. The reason for using a limiting reactant is that the elements and compounds react with each other in a balanced chemical equation according to the mole ratio between them. This reactant is the Limiting Reagent.
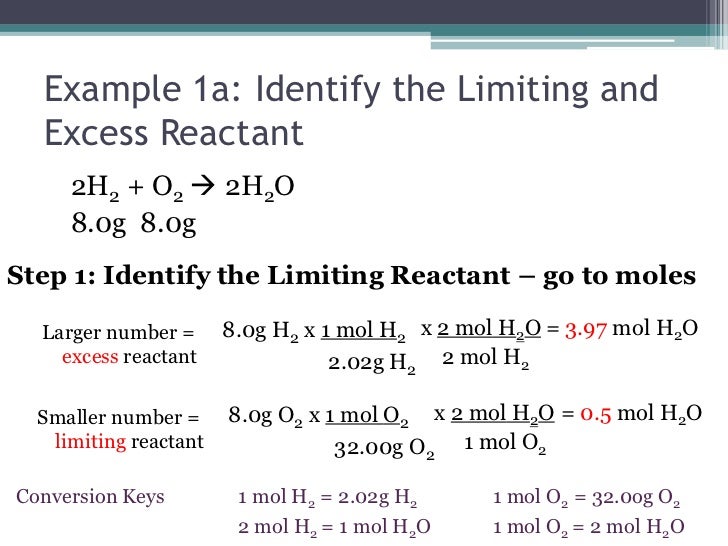 Chemistry I Honors Stoichiometry Limiting Reactant
Chemistry I Honors Stoichiometry Limiting Reactant
 Identify Limiting Reactants Maximum Produ Clutch Prep
Identify Limiting Reactants Maximum Produ Clutch Prep
 Limiting Reactant Example Problem 1 Edited Video Khan Academy
Limiting Reactant Example Problem 1 Edited Video Khan Academy
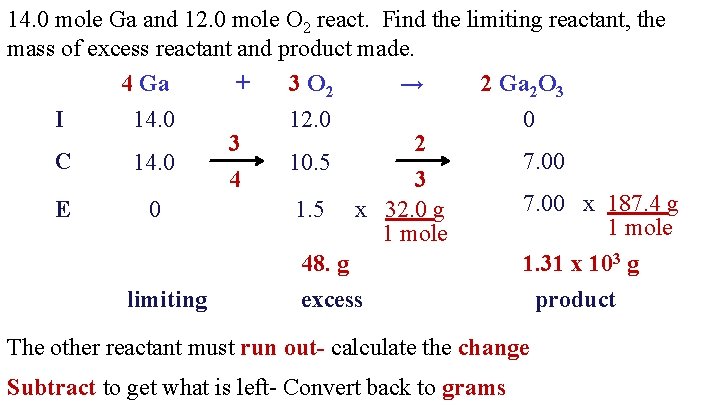 Limiting Reactants And Ice Charts Chemistry Cake You
Limiting Reactants And Ice Charts Chemistry Cake You
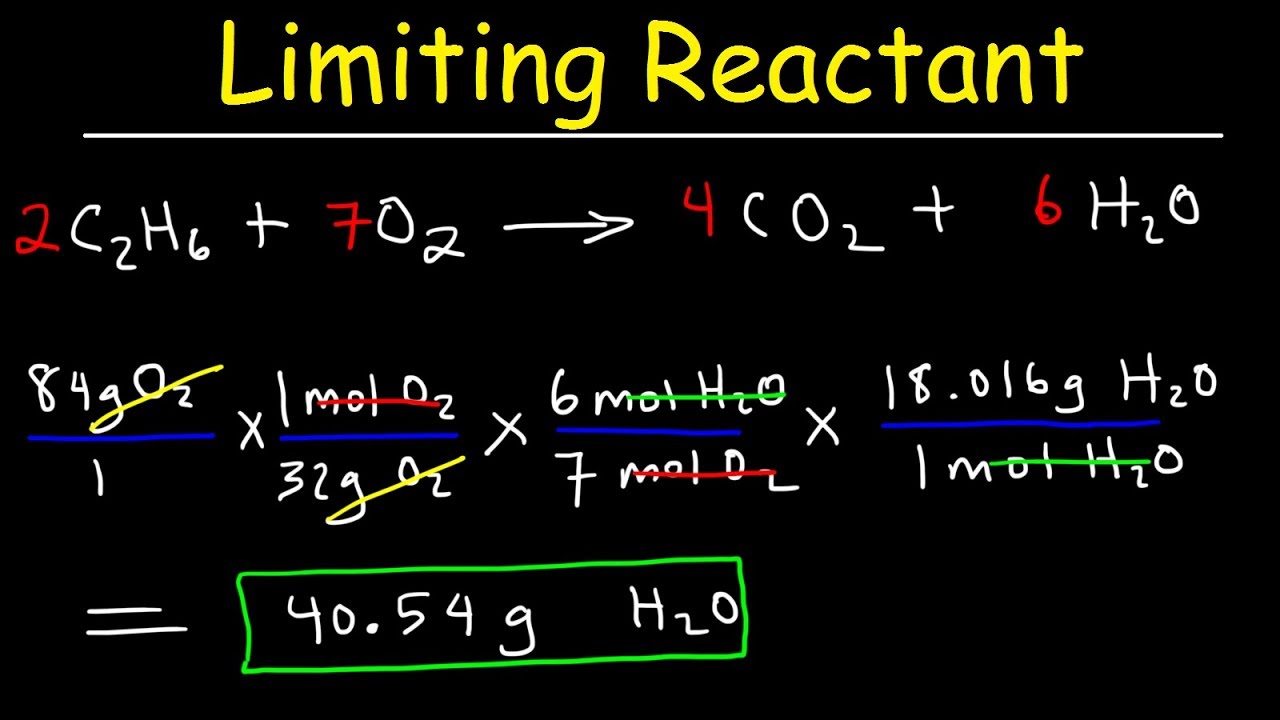 Limiting Reactant Practice Problems Youtube
Limiting Reactant Practice Problems Youtube
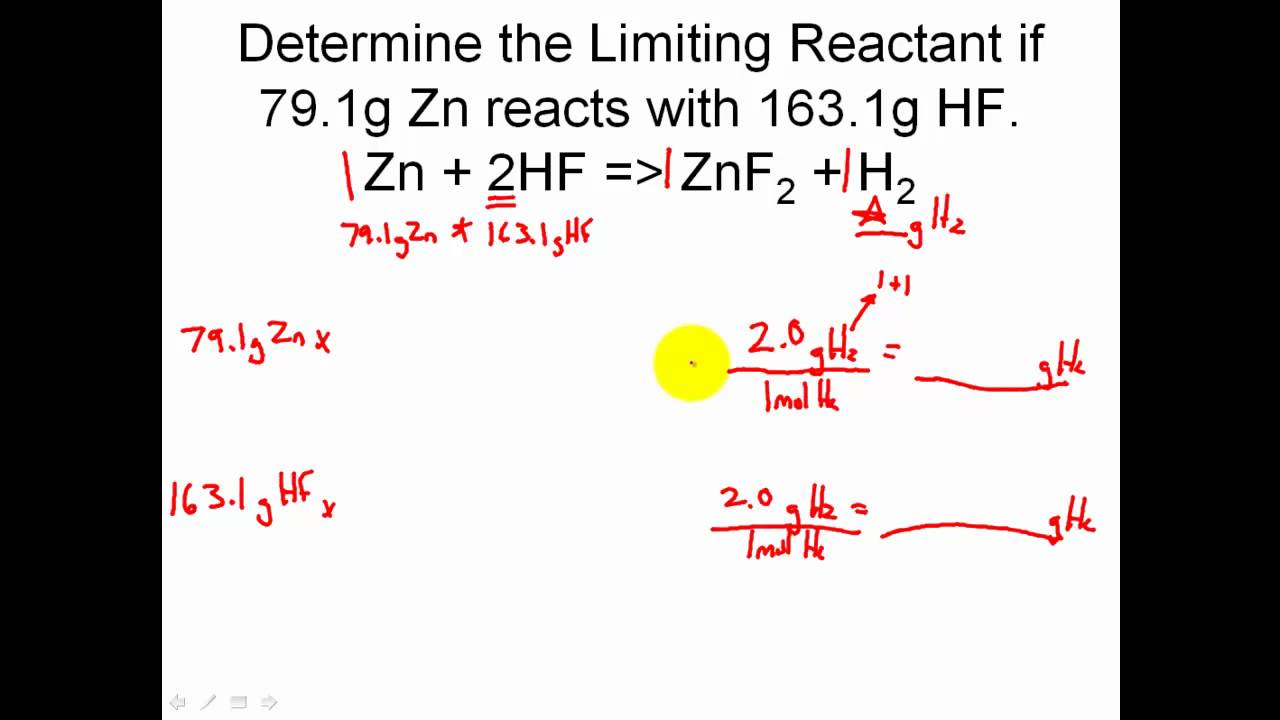 Stoichiometry Solving Limiting Reactant Problems In Stoichiometry Easy Youtube
Stoichiometry Solving Limiting Reactant Problems In Stoichiometry Easy Youtube
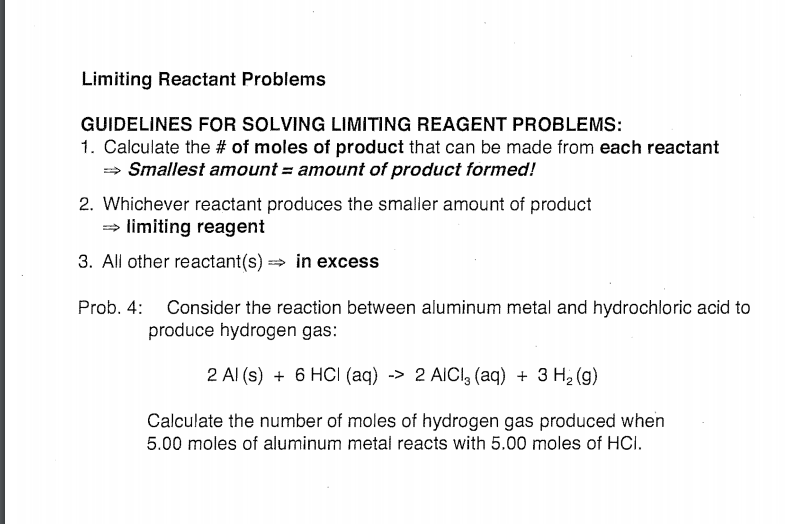
 Solved Limiting Reactant Problems Guidelines For Solving Chegg Com
Solved Limiting Reactant Problems Guidelines For Solving Chegg Com
 Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Apologia Chemistry School Work
Stoichiometry Limiting Reactant Excess Reactant Stoichiometry Moles Module 6 Learning Psychology Apologia Chemistry School Work
 Limiting Reactant Practice Problem Youtube
Limiting Reactant Practice Problem Youtube
 Chemistry I Honors Stoichiometry Limiting Reactant
Chemistry I Honors Stoichiometry Limiting Reactant
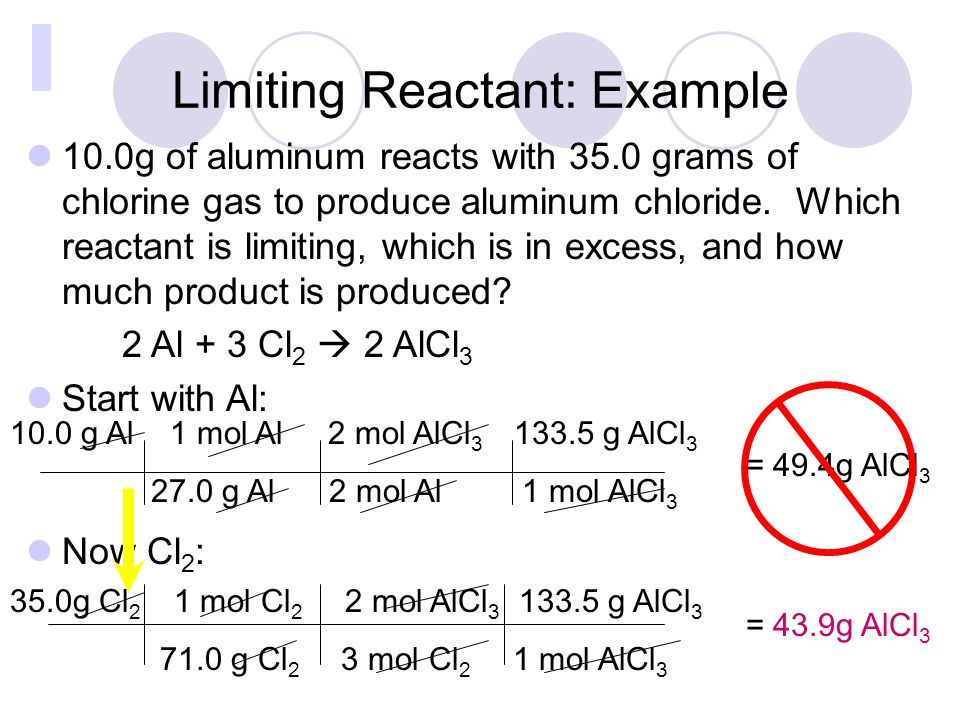 Limiting Reactants And Percent Yield Ppt Video Online Download
Limiting Reactants And Percent Yield Ppt Video Online Download
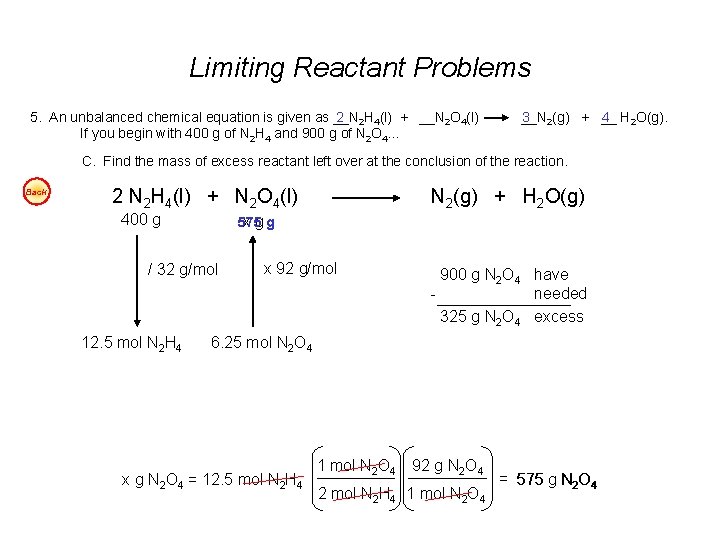 Visualizing Limiting Reactant 2 H 2 O 2
Visualizing Limiting Reactant 2 H 2 O 2
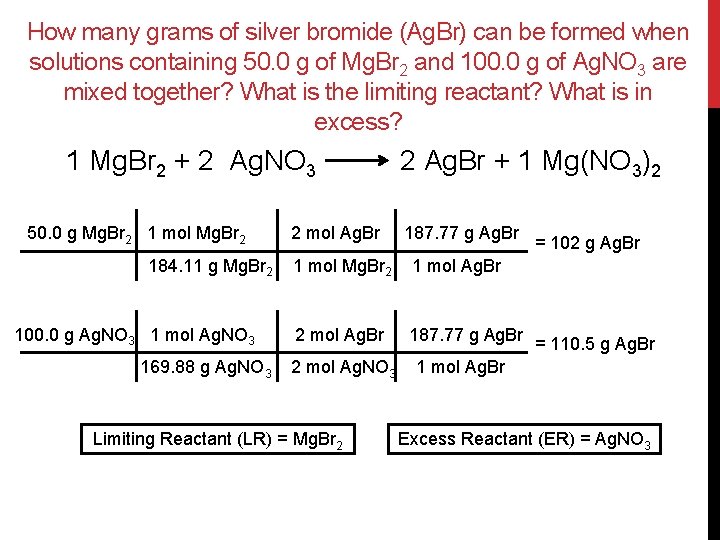 Limiting Reactant Limiting Reactant Limiting Reagent The Reactant
Limiting Reactant Limiting Reactant Limiting Reagent The Reactant
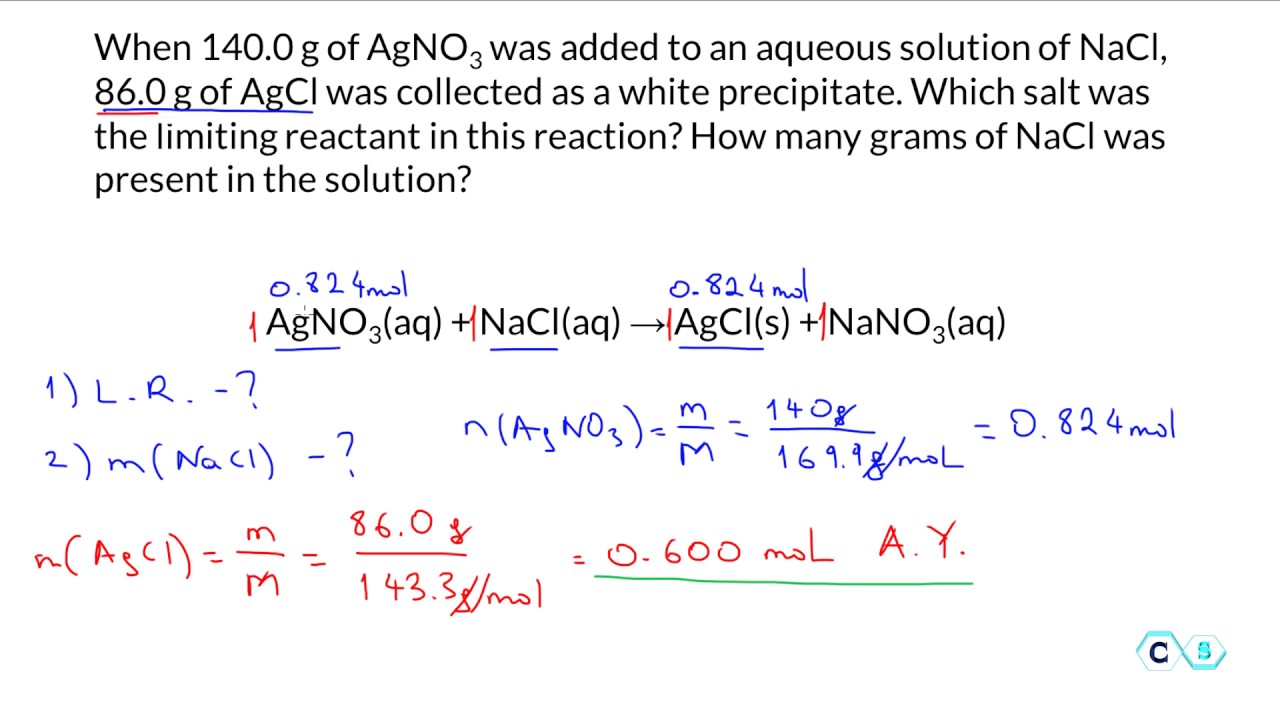 Limiting Reactant In The Stoichiometry Of Chemical Reactions
Limiting Reactant In The Stoichiometry Of Chemical Reactions
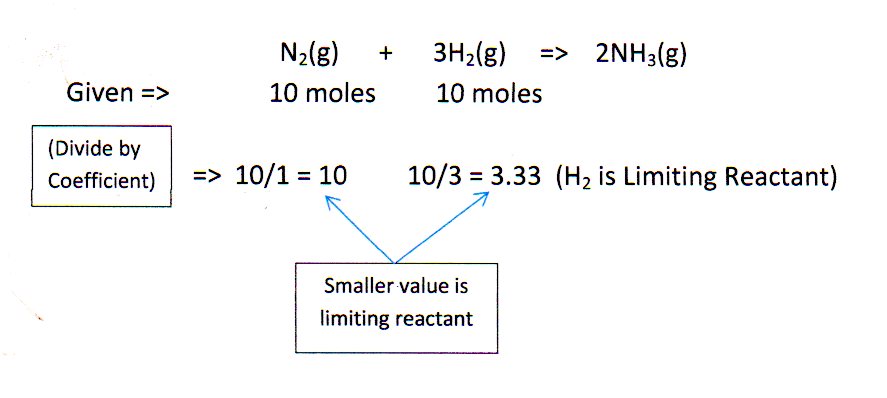 Limiting Reactants Rec Lessons Blendspace
Limiting Reactants Rec Lessons Blendspace
 How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
Limiting Reactants Chemistry 101
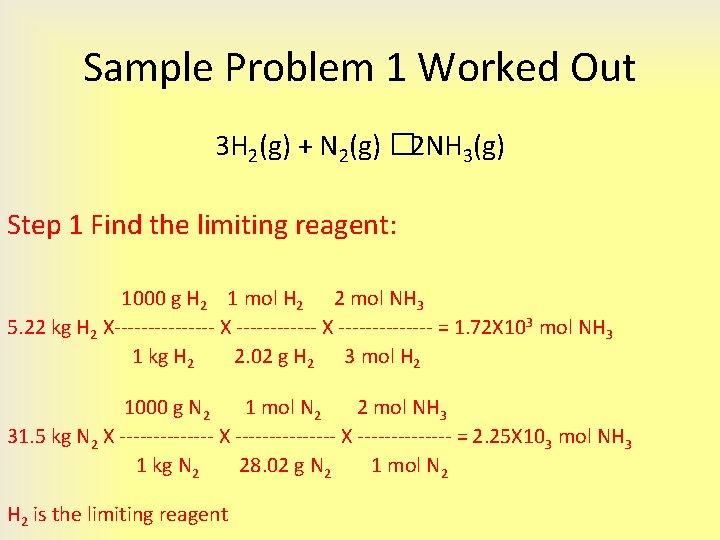 4 3 Limiting Reactant Theoretical Yield And Percent
4 3 Limiting Reactant Theoretical Yield And Percent
Post a Comment
Post a Comment